VESTAKEEP® Implantable PEEK and Medical Grade PEEK:
The World’s FIRST Implantable PEEK Filament
Modern Plastics is the authorized Evonik VESTAKEEP® distributor for North America.
Evonik has become the first company in the world to develop an implantable PEEK Filament for use in Fused Filament Fabrication of permanent implants.
VESTAKEEP® Applications:
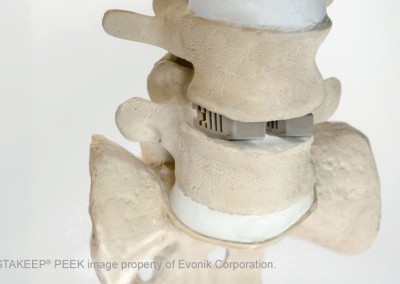
Common applications for Evonik VESTAKEEP® include PEEK spine implant and PEEK spinal cages, PEEK cranial implant, stents, heart valves, facial implants for facial bone fractures, access points, suture anchors, interference screws, small joints, and PEEK orthopedic implants.
Available in sheets, rods, resin, powder and filament.
VESTAKEEP ® PEEK Advantages and PEEK Properties:
- Biocompatibility
- Biostability
- Sterilization compatible
- Resistant to chemicals
- Modulus similar to bone
- Metal-free
- Wear comfort due to light weight and low thermal conductivity
- No x-ray artifacts and/or adjustable opacity
- Injection molding and extrusion compatible
- Low water absorption
- Easy to machine
- Good processability
- Lower stress-shielding effect

Shown: EVONIK 3D Implantable VESTAKEEP ® PEEK filament
Grades Offered:
- Implantable Grade Powder VESTAKEEP ® i2 P
- Implantable Grade Ultra-fine Powder VESTAKEEP ® UFP10
- Implantable Grade Powder VESTAKEEP ® i4 P
- Implantable Grade Resin Molding Granules VESTAKEEP ® i2 G
- Implantable Grade Resin Extrusion & Molding Granules VESTAKEEP ® i4 G
- Implantable Grade Resin Extrusion & Molding Rod VESTAKEEP ® i4 R
- Implantable Grade Resin Extrusion & Molding Plate VESTAKEEP ® i4 PL
- High Ductility Implantable Grade Granules VESTAKEEP ® i5 G
- High Ductility Implantable Grade Rod VESTAKEEP ® i5 R
- X-ray Visible Grade VESTAKEEP ® iC4506
- Medical 3D Printing Grade Filament VESTAKEEP ® i4 3DF
- Medical 3D Printing Test Grade Filament VESTAKEEP ® i4 3DF-T
- VESTAKEEP ® Dental Grades Available
Markets Served:
The following medical markets use VESTAKEEP® medical PEEK: Cranial-Maxillo-Facial, Cardiovascular, Pharmacy, Spine, Orthopedics, Sports Medicine, Neurological, and Extremities.
Evonik VESTAKEEP® (PEEK) Medical Polymers for permanent implant and short term contact
- 510(k) cleared in spine and other implant applications
- No contractual requirements
- No royalties or start up fees
- Stock inventory held in U.S.A.
- Forms: PEEK stock shapes in sheets, rods, plates, resin, powder, and filament
- One and three meter lengths
- Other stock shapes available
- Same day service, overnight shipping
- Environmentally Controlled and Monitored Storage Conditions
- Bar coded inventory
- Complete certifications and traceability
- Meets ASTM F2026 requirements
- Tested to ISO-10993 requirements
- Evonik Master Files for both the VESTAKEEP ® Implant grade resins and stock shapes
- 20 year record retention
- ISO 9001:2015 and ISO 13485:2016 Certified
- NDA documents available
Modern Plastics is now accepting orders for VESTAKEEP Implantable PEEK Filament.
We are offering a “Testing Grade” filament and a permanent implantable grade of PEEK filament.
For more information and pricing please contact Vince Griffin, Global Sales Manager for the Medical Plastics Division, at vgriffin@modernplastics.com.
Office: 800.243.9696, Ext. 205
cell: 203.260.7738
email: vgriffin@modernplastics.com
Search Our Plastics Database
Learn more about our robust inventory high performance plastics searchable by keyword, industry, manufacturer and shape.